Since May 1, 2021, China NMPA has implemented a safety system to collect the safety information of each cosmetics ingredients that has been listed in IECIC 2021. It requires cosmetics raw materials manufacturers necessary to submit the safety related information on the platform before the cosmetics registrant, filing person or domestic responsible person can fill in the raw material notification code during the cosmetics notification and registration.

The Raw material safety information can be submitted in two types:
- Cosmetics registrant, filing person(Brands) or Domestic responsible person: no notification code is generated
- Raw material manufacturer or authorized responsible person: a notification code will be generated
CosmeticsBridge: The benefit of cosmetics raw materials manufacturers to notify the products is that the ingredient only needs to be notified once and it can be used for all your clients. You don’t need to provide your client safety information over and over again.
Reporting Process of Cosmetic Raw Materials Safety Notification
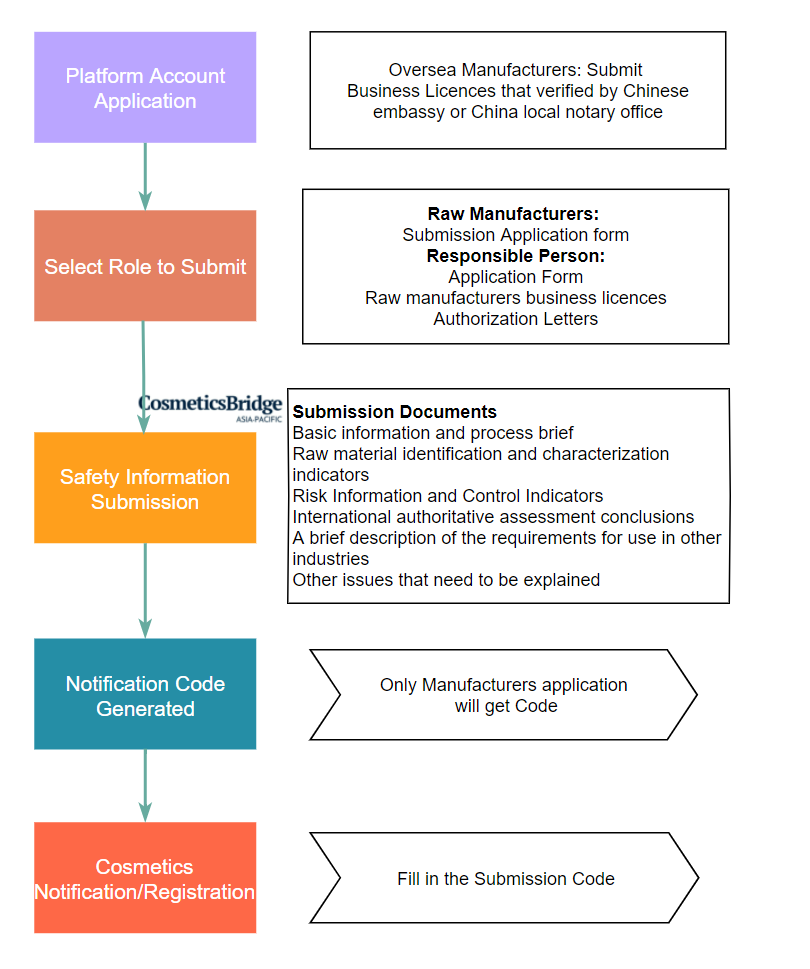
Milestones:
From January 1, 2022, When applying for cosmetics registration or filing, the cosmetics registrant and filing person shall provide safety-related information on raw materials with antiseptic, sunscreen, coloring, hair dyeing, freckle-removing and whitening functions.
From January 1, 2023, when applying for registration or filing, the cosmetics registrant and filing person shall provide safety-related information of all raw materials in accordance with the requirements of the “Regulations”. For cosmetics that have been registered or filed before, the registrant and filer shall provide additional safety-related information on all raw materials in the product formula before May 1, 2023.
It is not registration of new raw materials!
The purpose of the raw material safety submission is to ensure the safety of the ingredients that are allowed to be used in cosmetics(listed in IECIC 2021). The new cosmetic raw materials indicate the cosmetic raw material that has not been used in China cosmetics before and can not be found in IECIC 2021. Registration for before being used in cosmetics.
Overseas raw material manufacturers can apply the code directly!
Different from the registration of finished cosmetic products, overseas raw material manufacturers can directly fill in the report through the cosmetic raw material safety information platform, and receive the raw material notification code after submission.
Furthermore, manufacturers can authorize domestic responsible person enterprises to submit and routinely maintain raw material safety information. If there is authorization, after the authorized enterprise opens an account, it should submit the authorization letter issued by the raw material manufacturer. The authorization letter should specify the authorization relationship and scope of authorization, and only one company can be authorized for the same ingredient.
Contact us to request services:


